Tree Care: Nutrients and the Flow of Energy
Author
Published
1/1/2016
Target Grade Level / Age Range:
Grades 6-8
Estimated Time:
45 minutes
Purpose:
Students will understand the flow of energy in an environment using Christmas trees and the photosynthesis equation as the lens.
Materials:
- Scratch paper or science notebooks
- Scissors
- Whiteboard and markers
Essential Files (maps, charts, pictures, or documents)
Vocabulary
- Nutrients: substances that provide nourishment essential for growth and the maintenance of life
- Fertilizer: a substance added to soil or land to increase its fertility
- Deficiency: a lack or shortage
- Photosynthesis: the process by which green plants and some other organisms use sunlight to synthesize foods from carbon dioxide and water
- Molecule: a group of atoms bonded together, representing the smallest fundamental unit of a chemical compound that can take part in a chemical reaction
- Atom: the basic unit of a chemical element
- Equation: a statement that the values of two mathematical expressions are equal
- Law of Conservation of Mass: the principle that states that matter is never created nor destroyed
Background – Agricultural Connections
This lesson starts with a recap of what plants need to grow. There are many varieties of Christmas trees that have slightly different requirements, but the major things are sunlight, water, air, nutrients, and space. The specific climate zones, water requirements, soil types may vary variety to variety.
This lesson explores how plants get these things, specifically the nutrients necessary to build the physical plant material. There are 17 essential crop nutrients, including carbon, hydrogen, oxygen, nitrogen, phosphorus, potassium, sulfur, calcium, magnesium, boron, chlorine, copper, iron, manganese, molybdenum, nickel, and zinc. Carbon, hydrogen, and oxygen are absorbed the most through air and water. The rest primarily come from nutrients in the soil.
But how do these nutrients get to the soil? They can come from many places. Plants, insects, and microorganisms live and die in the soil and their decomposing structures return their nutrients back to the soil. Organic structures can also be added to soil, in the forms of compost or manure. Inorganic structures can also be added to the soil, in the forms of mined minerals like phosphorus, or synthesized nitrogen.
In the lesson, students will diagram the flow of energy between living and nonliving things in the Christmas tree’s environment. They will need to draw arrows between the tree and where the tree gets energy from (sun, water, soil, air), and also where the nutrients in the soil and air come from. Students should begin to understand through this activity that elements in the environment cycle from place to place. Elements and energy do not appear from nowhere, they are simply recycled throughout the environment.
Later in the lesson, students will use the photosynthesis equation as a more specific example of this phenomenon. Students will get a worksheet outlining the photosynthesis equation and will use paper atoms to see that all atoms are accounted for before and after the reaction takes place. This should reinforce the idea that elements don’t simply appear, they are recycled from other locations in the environment. This also ties into the Law of Conservation of Mass.
Interest Approach – Engagement
Start class with a bell-ringer question written on the white board stating, “What do Christmas trees need to grow? Where do they get those things?”
Have students raise their hands to share answers and thoughts. Capture ideas and responses on the board or a KWL chart.
Procedures
- Let’s review what plants like Christmas trees need to grow. What do they need?
- Sunlight
- Air
- Nutrients
- Water
- Climate/weather specifics
- Space
- Lack of disease, pest pressure, damaging weather events, etc.
- How do Christmas trees get what they need to grow?
- Explore what living things are made of.
- Plants are made of cellulose, which is carbon, hydrogen and oxygen
- DNA is made of nitrogen, hydrogen, oxygen, carbon, and phosphorus
- Other important elements include calcium, sulfur, and potassium
- Where do these things come from?
- Follow the flow of energy together as a class from the perspective of a Christmas tree. Draw a diagram on the board to illustrate the point as students go along.
- Example diagram below includes arrows from sun to plant life and arrows from soil to plant life to signify where they get nutrients and energy to grow. Animals like a deer and a fox eat plants to gain energy and leave manure, which contributes to soil nutrients. Finally, living organisms feed on decomposing organisms and eventually decompose themselves, all increasing the nutrients in the soil. What other elements are missing?
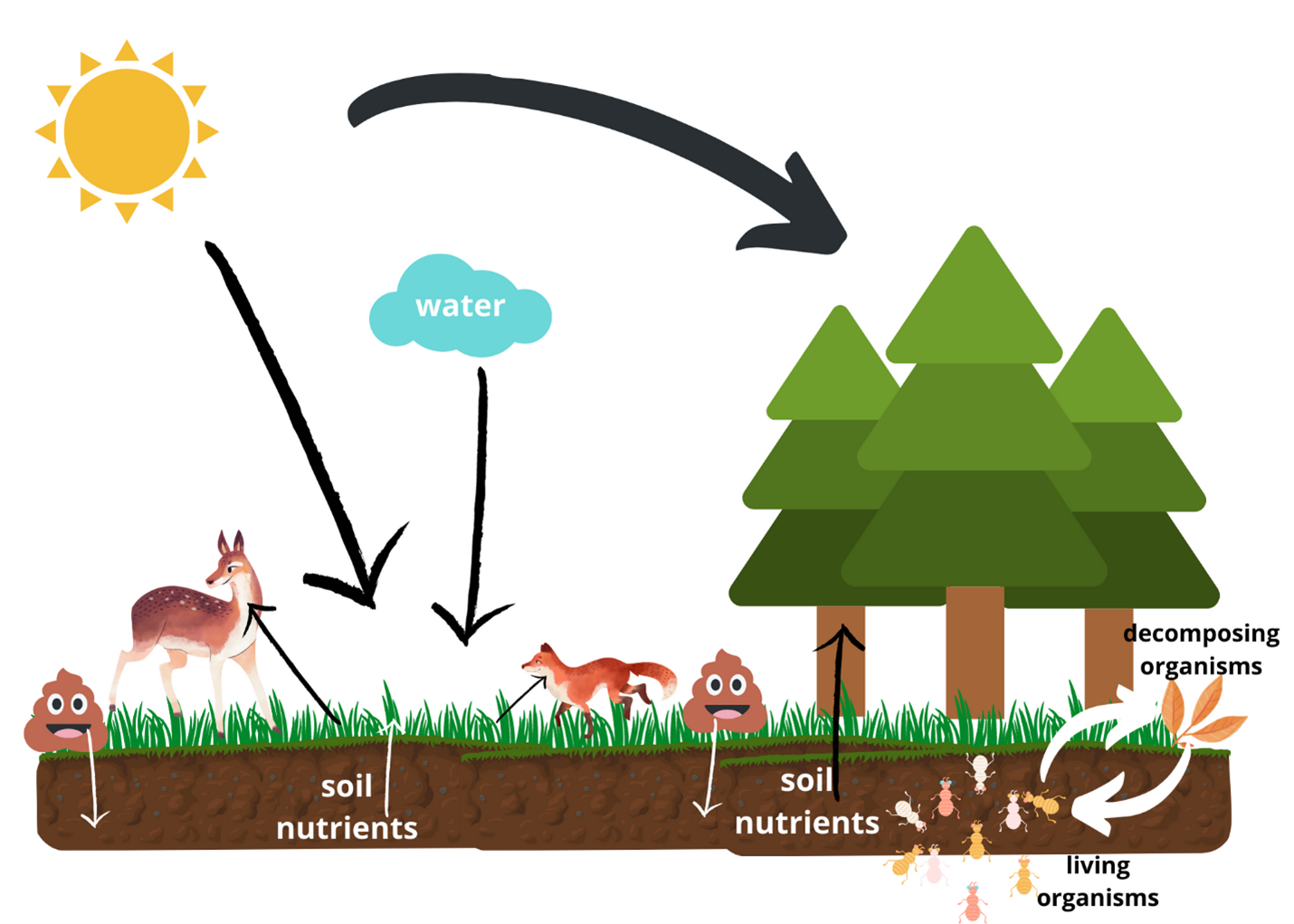
- Example diagram below includes arrows from sun to plant life and arrows from soil to plant life to signify where they get nutrients and energy to grow. Animals like a deer and a fox eat plants to gain energy and leave manure, which contributes to soil nutrients. Finally, living organisms feed on decomposing organisms and eventually decompose themselves, all increasing the nutrients in the soil. What other elements are missing?
- Follow the flow of energy together as a class from the perspective of a Christmas tree. Draw a diagram on the board to illustrate the point as students go along.
- Trees need certain elements to grow and survive. Where do they get those elements?
- Trees consume sunlight, water, and nutrients from the soil.
- Soils can be very different from place to place. Some soils have many nutrients and good soil structure. Other soils do not. Fertilizers can be added to soils to give extra nutrients to the soil. These nutrients are necessary in plant growth and can be depleted from the soil if fertilizers aren’t added over long periods of time.
- Are the elements infinite? Are the renewable? What happens when a tree uses nutrients?
- Elements in the ecosystem aren’t infinite, but instead they cycle throughout the environment and get reused over and over again. When the tree makes energy, it gives off oxygen, which we breathe. When the tree dies, it can be returned to the soil to add more nutrients for the next trees to use. Fertilizers can also be mined, synthesized from the air, or collected (think compost and manure) to be applied to land to replenish nutrients.
- At this time, have students take out a science notebook or hand out scratch paper for them to use. Instruct them to create their own diagram of this including these elements.
- Instruct them to draw key pieces of the environment that use and redistribute energy in the environment.
- Instruct them to draw arrows showing the energy flow from item to item. For example, an arrow from the soil into the plant to show that the plant gains nutrients form the soil.
- Instruct them further to label items as needed for clarity.
- Give students a few minutes to draw their diagram and share/recap as a whole group.
- At this time, have students take out a science notebook or hand out scratch paper for them to use. Instruct them to create their own diagram of this including these elements.
- Elements, nutrients, and energy are all kind of broad terms to describe generally what’s going on in the environment, but let’s get more specific with an example to help explain.
- The Law of Conservation of Energy states that matter is neither created nor destroyed. To demonstrate that, let’s explore photosynthesis.
- Photosynthesis occurs when a plant takes in carbon dioxide, water, and sunlight and the plant makes glucose and oxygen.
- When that reaction happens, no singular element is left out. Everything remains accounted for, even if they show up in different ways.
- Hand out the photosynthesis equation worksheets to students and make sure each student also has scissors.
- Tell students that they will be walking through this themselves with this activity. Tell students to begin cutting out each of the small circles on the second page only of the worksheet. The first page needs to remain intact. Give students a few minutes to work on this.
- After student have their atoms cut out, walk through the worksheet together. Each of their tiny atoms matches up with an atom in the first half of the equation (top half of the page). Have students start placing their atoms in the correct locations on the top half of the page (with carbon dioxide and water).
- Explain to students while they do this that plants get carbon dioxide from the air and water from their roots. They absorb things in the environment that help them grow. They also absorb sunlight through their leaves, which helps the chemical reaction start. What is the chemical reaction? Photosynthesis.
- Have everyone wiggle their fingers at their paper or otherwise signify that the chemical reaction has happened and have them start moving each atom one by one into either the glucose or O2 molecules at the bottom half of the paper.
- Point out to students that each atom is accounted for. There shouldn’t be any leftover atoms from the beginning of the equation to the end of the equation. That’s because matter is never created nor destroyed, according to the Law of Conservation of Mass. Things more or less cycle through the environment, being put to use in different ways throughout space and time.
- When a Christmas tree grows, it uses carbon dioxide from the air, water, and sunlight to photosynthesize and create energy. It gains other nutrients from the soil. When the Christmas tree is cut, it is then enjoyed by a family until it starts to decay, at which point it can then be returned to the earth and be broken down into nutrients to help other trees grow.
- To wrap up, have students glue the atoms onto the glucose and O2 molecules and include the full worksheet in a science notebook, or hang them in the room.
Did you know? (Ag facts)
- An acre of Christmas trees produces the daily oxygen requirements for 18 people.
- Recycled Christmas trees have been used to make sand and soil erosion barriers and placed in ponds for fish shelters.
Extension Activities
- Assign students to an atom and recreate chemical reactions by moving around the room and into different “molecules”.
- Have students create 3D models of a specific ecosystem and demonstrate where energy is cycling within that ecosystem.
- Have a virtual or live meeting with a Christmas tree farmer to discuss how photosynthesis plays a role in their farming operation.
Sources/Credits
- This publication or project was supported by the U.S. Department of Agriculture’s (USDA) Agricultural Marketing Service through grant 21SCBPIA1013 Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the USDA.
- https://serc.carleton.edu/eslabs/carbon/1a.html
- https://sciencing.com/make-biology-flow-chart-6974029.html
- https://www.compoundchem.com/2015/03/24/dna/
- https://web.extension.illinois.edu/trees/facts.cfm
Author(s)
Chrissy Rhodes
Organization Affiliation
Iowa Agriculture Literacy Foundation
Agriculture Literacy Outcomes
- Theme 4: Science, Technology, Engineering and Math:
- T4.6-8.b: Describe how biological processes influence and are leveraged in agricultural production and processing (e.g., photosynthesis, fermentation, cell division, heredity/genetics, nitrogen fixation)
Iowa Core Standards
- Science
- MS-ESS2-1. Develop a model to describe the cycling of Earth’s materials and the flow of energy that drives this process.
- MS-PS1-1. Develop models to describe the atomic composition of simple molecules and extended structures.
- MS-PS1-5. Develop and use a model to describe how the total number of atoms does not change in a chemical reaction and thus mass is conserved.
- MS-LS1-6. Construct a scientific explanation based on evidence for the role of photosynthesis in the cycling of matter and flow of energy into and out of organisms.